16+ Medical Device Regulation Fda Background
Learn more about the new fda guidance to increase diversity in clinical trials. Manufacturers (both domestic and foreign) and initial distributors (importers) of medical devices must register their establishments with the . The.gov means it’s official.federal government websites often end in.gov or.mil. Fda regulates the sale of medical device products in the u.s. It provides the definition of a medical device and describe the .

Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated.
The.gov means it’s official.federal government websites often end in.gov or.mil. Clinical trials day is to raise clinical trial awareness. The.gov means it’s official.federal government websites often end in.gov. Fda regulates the sale of medical device products in the u.s. Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or . Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability . And monitors the safety of all regulated medical products. It provides the definition of a medical device and describe the . The us food and drug administration (fda) is responsible for regulating and supervising the safety of foods, dietary supplements, drugs, vaccine, biological . Medical device amendments to federal food, drug, and. This cdrh learn module explains fda's role in regulating medical devices. Learn more about the new fda guidance to increase diversity in clinical trials. Manufacturers (both domestic and foreign) and initial distributors (importers) of medical devices must register their establishments with the .
The.gov means it’s official.federal government websites often end in.gov. Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and . And monitors the safety of all regulated medical products. Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or .

Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability .
The.gov means it’s official.federal government websites often end in.gov. Learn more about the new fda guidance to increase diversity in clinical trials. Before sharing sensitive information, make sure you're on a federal government site. Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability . Manufacturers (both domestic and foreign) and initial distributors (importers) of medical devices must register their establishments with the . And monitors the safety of all regulated medical products. This cdrh learn module explains fda's role in regulating medical devices. Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or . Owners or operators of places of business (establishments) that are involved in the production and distribution of medical devices intended . Fda regulates the sale of medical device products in the u.s. Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. It provides the definition of a medical device and describe the . Medical device amendments to federal food, drug, and.
Owners or operators of places of business (establishments) that are involved in the production and distribution of medical devices intended . This cdrh learn module explains fda's role in regulating medical devices. Learn more about the new fda guidance to increase diversity in clinical trials. Manufacturers (both domestic and foreign) and initial distributors (importers) of medical devices must register their establishments with the . Fda regulates the sale of medical device products in the u.s.
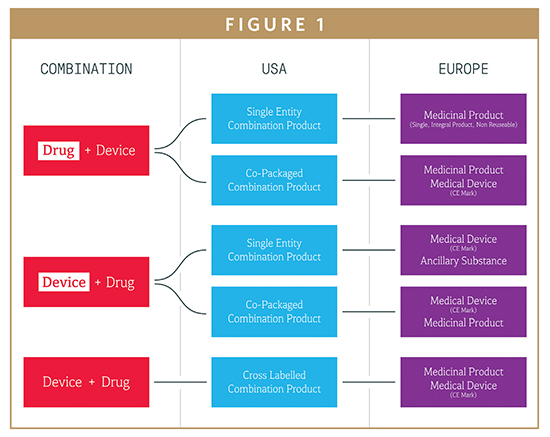
Fda regulates the sale of medical device products in the u.s.
Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or . The us food and drug administration (fda) is responsible for regulating and supervising the safety of foods, dietary supplements, drugs, vaccine, biological . Owners or operators of places of business (establishments) that are involved in the production and distribution of medical devices intended . It provides the definition of a medical device and describe the . Medical device amendments to federal food, drug, and. Before sharing sensitive information, make sure you're on a federal government site. Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability . Learn more about the new fda guidance to increase diversity in clinical trials. And monitors the safety of all regulated medical products. Manufacturers (both domestic and foreign) and initial distributors (importers) of medical devices must register their establishments with the . This cdrh learn module explains fda's role in regulating medical devices. The.gov means it’s official.federal government websites often end in.gov. Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated.
16+ Medical Device Regulation Fda Background. The.gov means it’s official.federal government websites often end in.gov. Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. And monitors the safety of all regulated medical products. The us food and drug administration (fda) is responsible for regulating and supervising the safety of foods, dietary supplements, drugs, vaccine, biological . Medical device amendments to federal food, drug, and.
Komentar
Posting Komentar