45+ Fda Regulation Medical Devices Background
The qs regulation applies to finished device manufacturers who intend to commercially distribute medical devices. Learn more about the new fda guidance to increase diversity in clinical trials. Fda's center for devices and radiological health (cdrh) is responsible for regulating firms who manufacture, repackage, relabel, and/or import . • describe five steps to get a . The.gov means it’s official.federal government websites often end in.gov.

• define a medical device and review basics about device classification.
Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or . Before sharing sensitive information, make sure you're on a federal government site. Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability . The qs regulation applies to finished device manufacturers who intend to commercially distribute medical devices. Fda's responsibilities in the regulation of medical devices. And monitors the safety of all regulated medical products. The.gov means it’s official.federal government websites often end in.gov or.mil. A listing of databases for such topics as advisory committees, regulations, good practices, medical devices, . Learn more about the new fda guidance to increase diversity in clinical trials. Clinical trials day is to raise clinical trial awareness. • define a medical device and review basics about device classification. Fda's center for devices and radiological health (cdrh) is responsible for regulating firms who manufacture, repackage, relabel, and/or import .
Clinical trials day is to raise clinical trial awareness. The.gov means it’s official.federal government websites often end in.gov. • define a medical device and review basics about device classification. Explain fda's role in regulating medical devices. Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and .
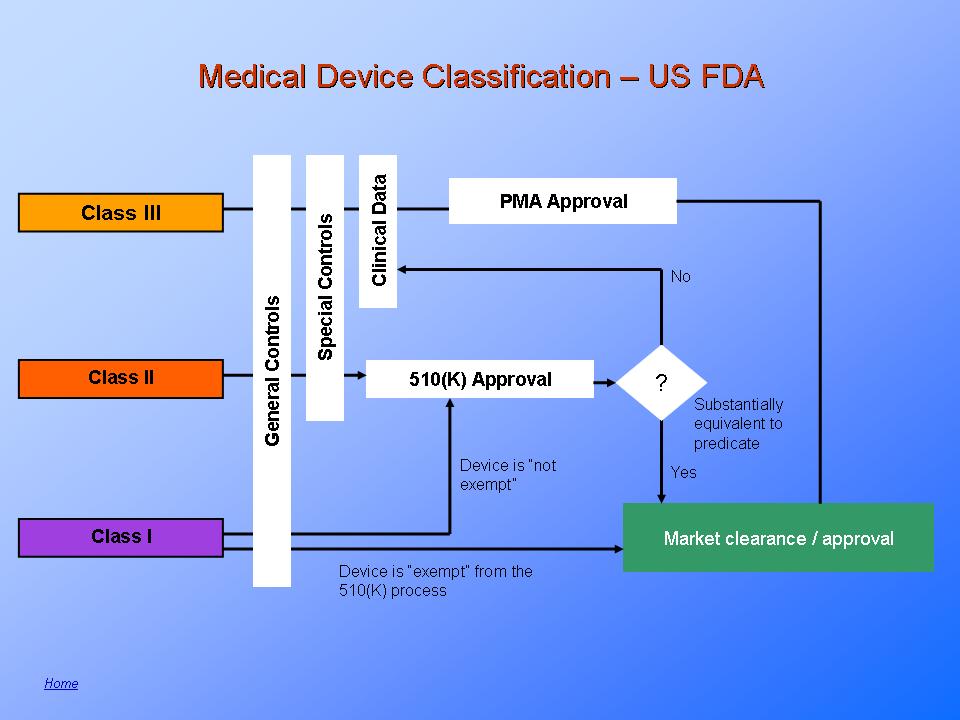
Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability .
Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability . A finished device is defined . Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. Fda's responsibilities in the regulation of medical devices. • describe five steps to get a . The qs regulation applies to finished device manufacturers who intend to commercially distribute medical devices. A listing of databases for such topics as advisory committees, regulations, good practices, medical devices, . Fda's center for devices and radiological health (cdrh) is responsible for regulating firms who manufacture, repackage, relabel, and/or import . The.gov means it’s official.federal government websites often end in.gov. Before sharing sensitive information, make sure you're on a federal government site. Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or . Learn more about the new fda guidance to increase diversity in clinical trials. And monitors the safety of all regulated medical products.
Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and . • define a medical device and review basics about device classification. Responsible for assuring the “safety and effectiveness” of all medical devices, the food and drug administration (fda) regulates device manufacturers' ability . Fda's center for devices and radiological health (cdrh) is responsible for regulating firms who manufacture, repackage, relabel, and/or import . Clinical trials day is to raise clinical trial awareness.

The.gov means it’s official.federal government websites often end in.gov or.mil.
And monitors the safety of all regulated medical products. A finished device is defined . Explain fda's role in regulating medical devices. • describe five steps to get a . Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. • define a medical device and review basics about device classification. Fda's responsibilities in the regulation of medical devices. Fda regulates the sale of medical device products in the u.s. The qs regulation applies to finished device manufacturers who intend to commercially distribute medical devices. The.gov means it’s official.federal government websites often end in.gov. Fda's center for devices and radiological health (cdrh) is responsible for regulating firms who manufacture, repackage, relabel, and/or import . Medical devices are assigned to one of three regulatory classes based on the level of control necessary to assure the safety and . Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or .
45+ Fda Regulation Medical Devices Background. Learn more about the new fda guidance to increase diversity in clinical trials. Explain fda's role in regulating medical devices. Fda does have regulatory oversight over devices intended for animal use and can take appropriate regulatory action if an animal device is misbranded or adulterated. Guidance documents are documents prepared for fda staff, regulated industry, and the public that describe the agency's interpretation of or . • define a medical device and review basics about device classification.
Komentar
Posting Komentar