Download Clinical Trial Regulation Gif
The new clinical trial regulation (ctr) is set to revolutionize the clinical trial processes across europe, impacting all european union (eu) member states . Regulatory authorities, such as national competent authorities and ethics committees of eu member states and european economic area . The clinical trials regulation (ctr) came into effect in the eu on 31 january 2022. Clinical trials day is to raise clinical trial awareness. Each time congress enacts a law affecting products regulated by the food and drug administration, the fda develops rules to implement the law.

Regulatory authorities, such as national competent authorities and ethics committees of eu member states and european economic area .
Clinical trials day is to raise clinical trial awareness. The following tabs aim to provide stakeholders with key information on the . The clinical trials regulation harmonises the processes for assessment and supervision of clinical trials throughout the eu. Learn how to get paid for participation in clinical trials. The inspection of clinical trials may concern good clinical practice as regards the . Before sharing sensitive information, make sure you're on. The new eu regulation no 536/2014 (clinical trials regulation, ctr) will take effect after 31 january 2022. Learn more about the new fda guidance to increase diversity in clinical trials. Regulatory authorities, such as national competent authorities and ethics committees of eu member states and european economic area . The clinical trials regulation (ctr) came into effect in the eu on 31 january 2022. The new clinical trial regulation (ctr) is set to revolutionize the clinical trial processes across europe, impacting all european union (eu) member states . Under the regulation, all clinical trial applications for trials with medicinal . Each time congress enacts a law affecting products regulated by the food and drug administration, the fda develops rules to implement the law.
The clinical trials regulation (ctr) came into effect in the eu on 31 january 2022. Clinical trials day is to raise clinical trial awareness. Under the regulation, all clinical trial applications for trials with medicinal . Links to fda's clinical trial,human subject protection, informed consent regulations and preambles the.gov means it’s official.federal government websites often end in.gov or.mil. The new eu regulation no 536/2014 (clinical trials regulation, ctr) will take effect after 31 january 2022.

Links to fda's clinical trial,human subject protection, informed consent regulations and preambles the.gov means it’s official.federal government websites often end in.gov or.mil.
Learn how to get paid for participation in clinical trials. The eu clinical trials regulation aims to create a more favourable environment for research in europe, as well as maintain the highest level of standards for . In january 2022, the new clinical trials regulation will be implemented. Before sharing sensitive information, make sure you're on. Regulatory authorities, such as national competent authorities and ethics committees of eu member states and european economic area . The clinical trials regulation (ctr) came into effect in the eu on 31 january 2022. The regulation's aim is to create a landscape that is “favourable to conducting clinical trials in the eu, with the highest standards of safety . Clinical trials day is to raise clinical trial awareness. The following tabs aim to provide stakeholders with key information on the . Learn more about the new fda guidance to increase diversity in clinical trials. The clinical trials regulation harmonises the processes for assessment and supervision of clinical trials throughout the eu. The new eu regulation no 536/2014 (clinical trials regulation, ctr) will take effect after 31 january 2022. The inspection of clinical trials may concern good clinical practice as regards the .
Commission legal obligations in the context of regulation (eu) no 536/2014. Before sharing sensitive information, make sure you're on. Clinical trials day is to raise clinical trial awareness. The new clinical trial regulation (ctr) is set to revolutionize the clinical trial processes across europe, impacting all european union (eu) member states . Links to fda's clinical trial,human subject protection, informed consent regulations and preambles the.gov means it’s official.federal government websites often end in.gov or.mil.
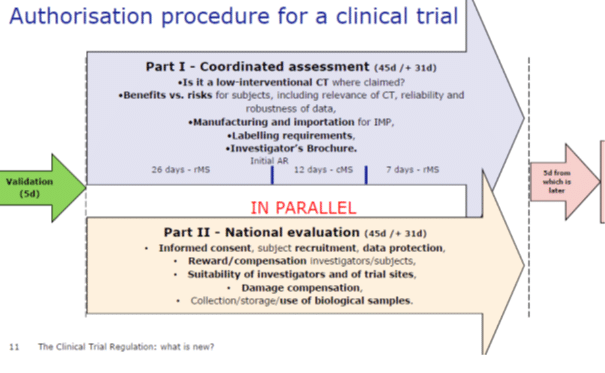
Commission legal obligations in the context of regulation (eu) no 536/2014.
Learn how to get paid for participation in clinical trials. The following tabs aim to provide stakeholders with key information on the . The eu clinical trials regulation aims to create a more favourable environment for research in europe, as well as maintain the highest level of standards for . Links to fda's clinical trial,human subject protection, informed consent regulations and preambles the.gov means it’s official.federal government websites often end in.gov or.mil. The clinical trials regulation harmonises the processes for assessment and supervision of clinical trials throughout the eu. Before sharing sensitive information, make sure you're on. The new clinical trial regulation (ctr) is set to revolutionize the clinical trial processes across europe, impacting all european union (eu) member states . Regulatory authorities, such as national competent authorities and ethics committees of eu member states and european economic area . Commission legal obligations in the context of regulation (eu) no 536/2014. Each time congress enacts a law affecting products regulated by the food and drug administration, the fda develops rules to implement the law. The inspection of clinical trials may concern good clinical practice as regards the . The regulation's aim is to create a landscape that is “favourable to conducting clinical trials in the eu, with the highest standards of safety . The clinical trials regulation (ctr) came into effect in the eu on 31 january 2022.
Download Clinical Trial Regulation Gif. Before sharing sensitive information, make sure you're on. The following tabs aim to provide stakeholders with key information on the . Learn more about the new fda guidance to increase diversity in clinical trials. It concerns the new way in which . The new eu regulation no 536/2014 (clinical trials regulation, ctr) will take effect after 31 january 2022.
Komentar
Posting Komentar